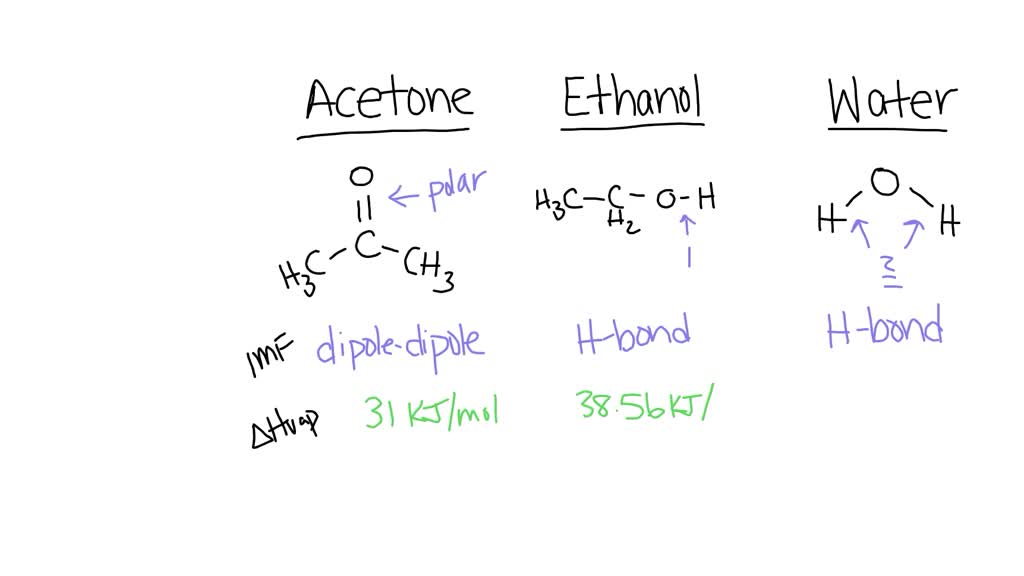
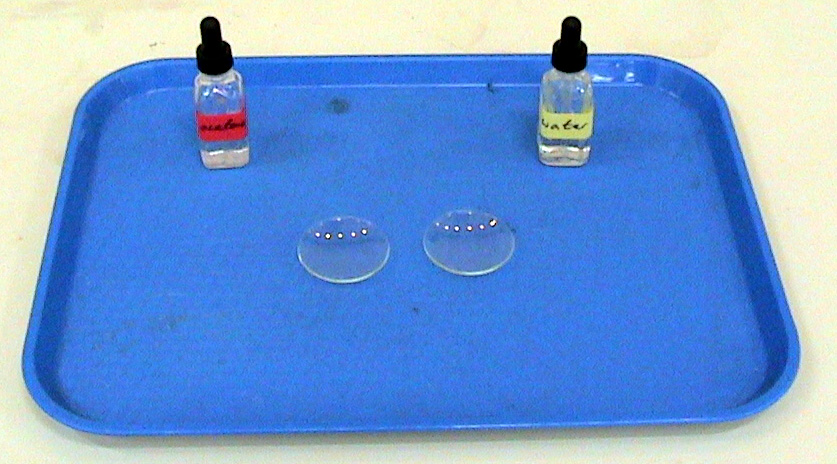
I have acetone in my hand, it evaporates so fast. Why doesn't the acetone evaporate when it is inside a bottle (my point is evaporation rate is slow)? - Quora

Evaporation rate depending on the inlet temperature for acetone/water... | Download Scientific Diagram

Acetone droplet temperature during droplet evaporation in experimental... | Download Scientific Diagram

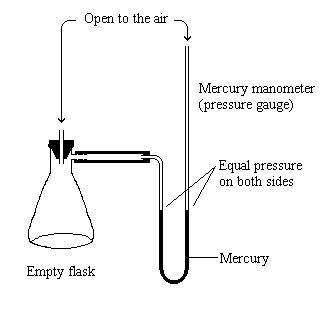
experimental chemistry - How can evaporation from a wash bottle be prevented? - Chemistry Stack Exchange

Evaporating sample mass vs. time dependence, and Acetone evaporation... | Download Scientific Diagram

a) Evaporation of acetone and isopropyl alcohol (IPA) and addition of... | Download Scientific Diagram

Evaporation monitoring curves of Isopropyl Alcohol, Acetone and Ethanol. | Download Scientific Diagram