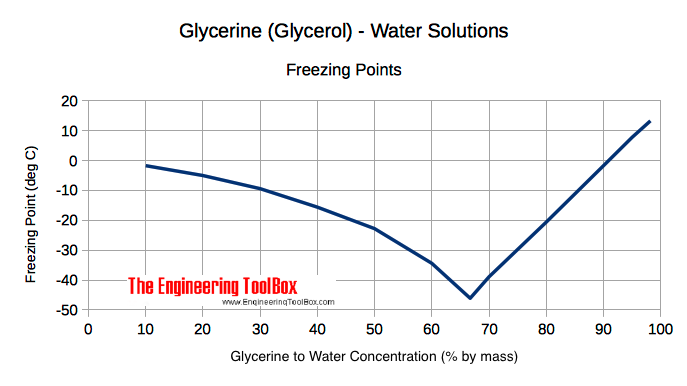
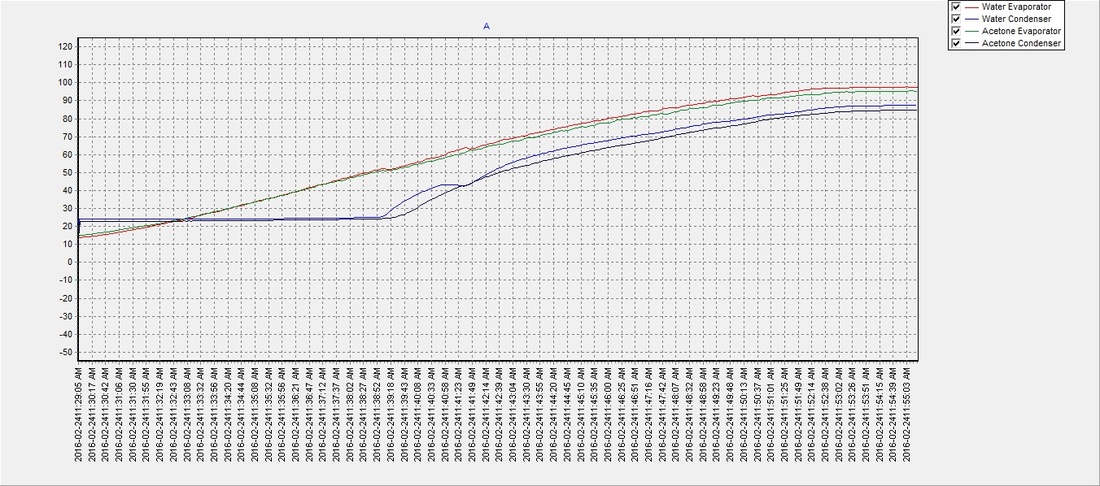
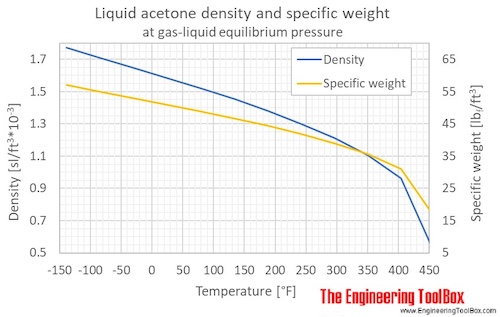
thermodynamics - What mixing ratio of ethanol and acetone has the lowest freezing point? - Chemistry Stack Exchange
What is the boiling point (1atm) of a solution 116g of acetone (Me 58) and 72g of water (Me 18)? - Quora
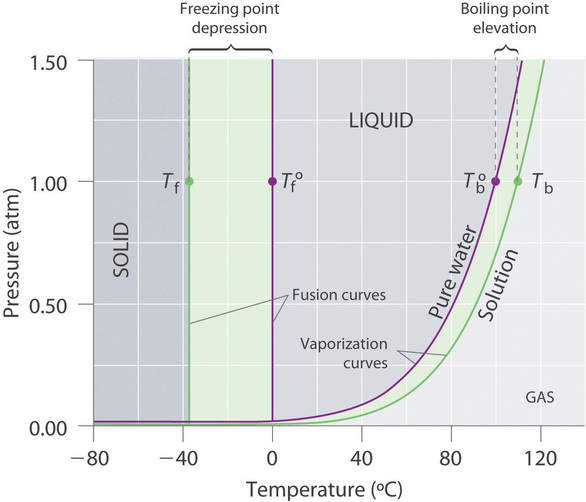
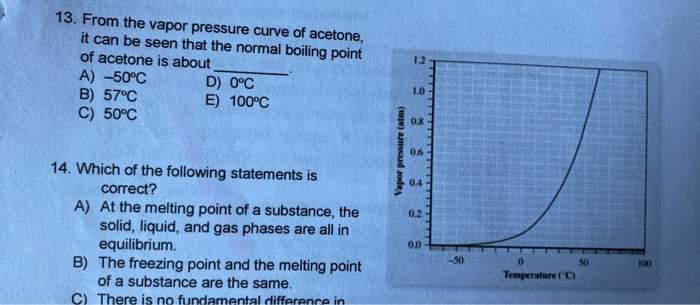
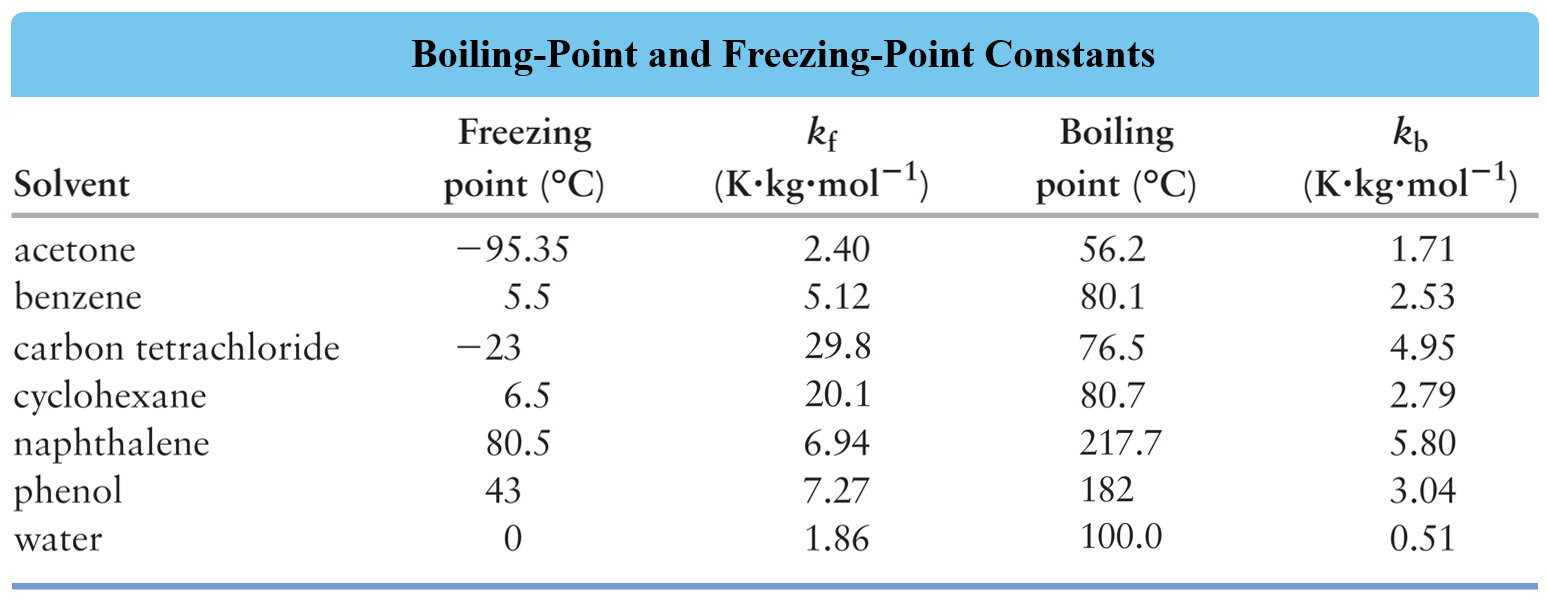
13.8: Freezing-Point Depression and Boiling-Point Elevation of Nonelectrolyte Solutions - Chemistry LibreTexts

1-Butanol Separation from Aqueous Acetone-Butanol-Ethanol (ABE) Solutions by Freeze Concentration | Crystal Growth & Design
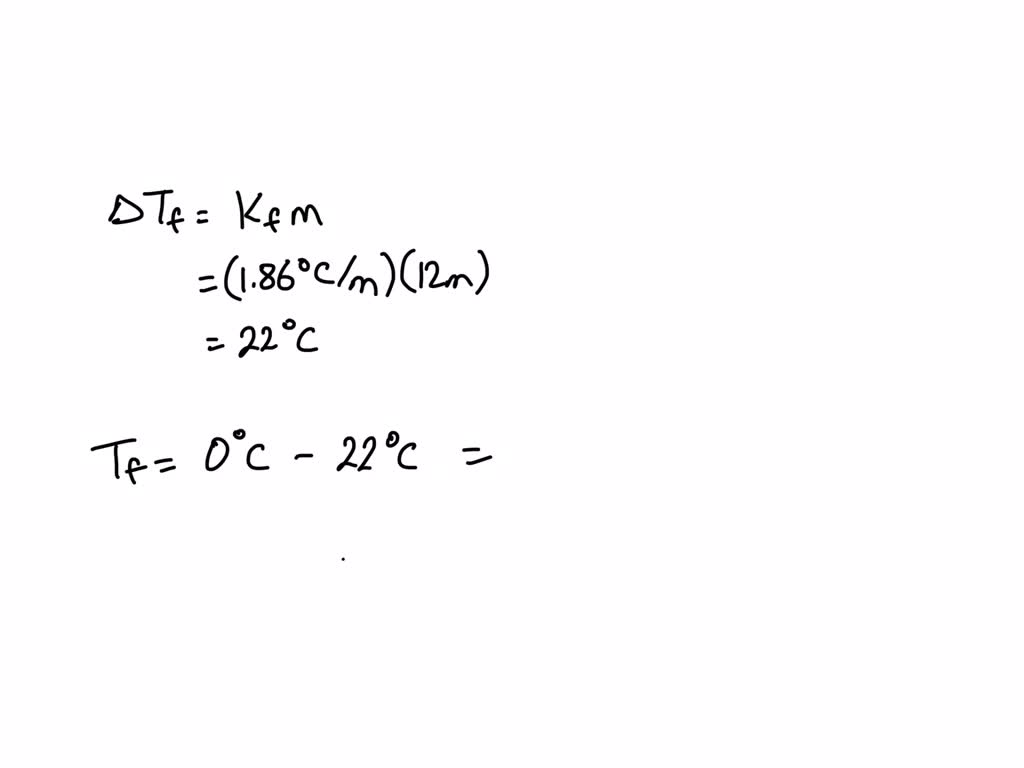
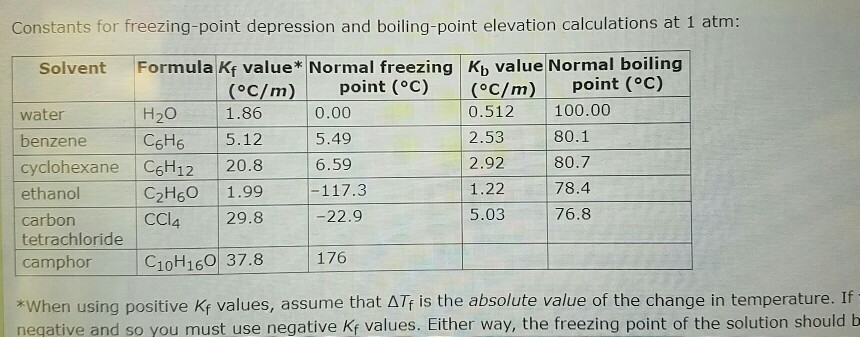
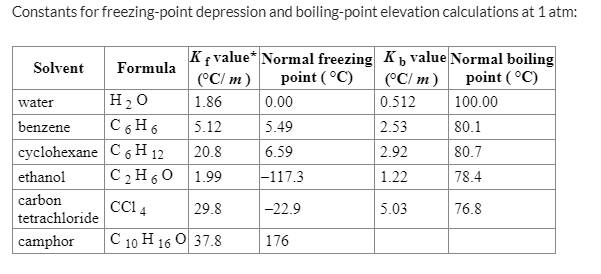
SOLVED: Calculate the freezing point of a 12.00 m aqueous solution of acetone. Freezing point constants can be found in the list of colligative constants. T f = ° C
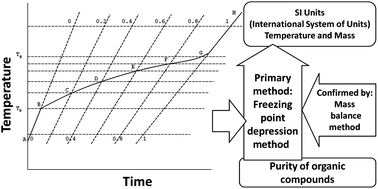
Development of purity certified reference materials for methanol, ethanol, acetonitrile, acetone, ethyl acetate and n-hexane by freezing point depression - Analytical Methods (RSC Publishing)

1-Butanol Separation from Aqueous Acetone-Butanol-Ethanol (ABE) Solutions by Freeze Concentration | Crystal Growth & Design
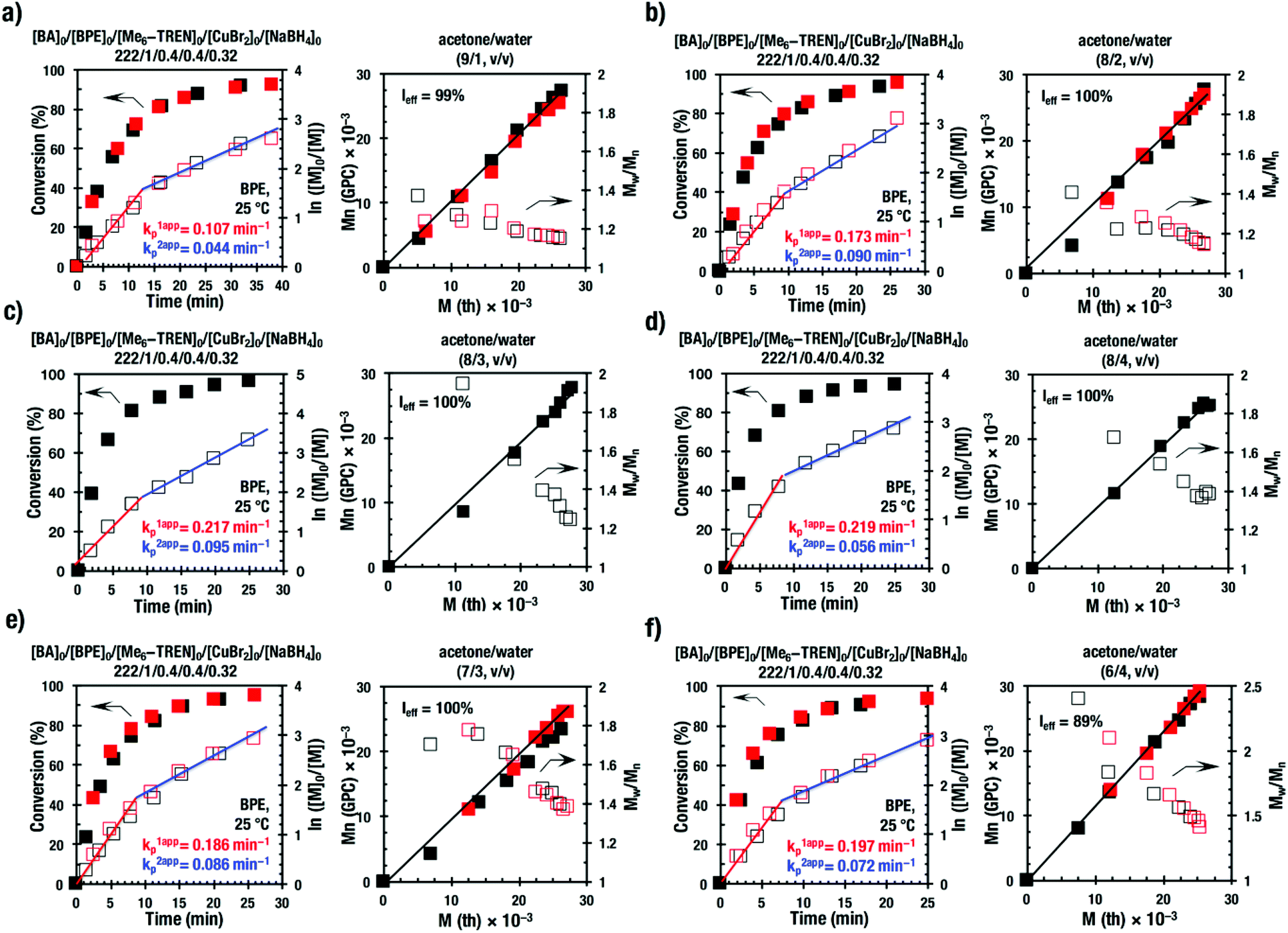
Acetone–water biphasic mixtures as solvents for ultrafast SET-LRP of hydrophobic acrylates - Polymer Chemistry (RSC Publishing) DOI:10.1039/C7PY00557A
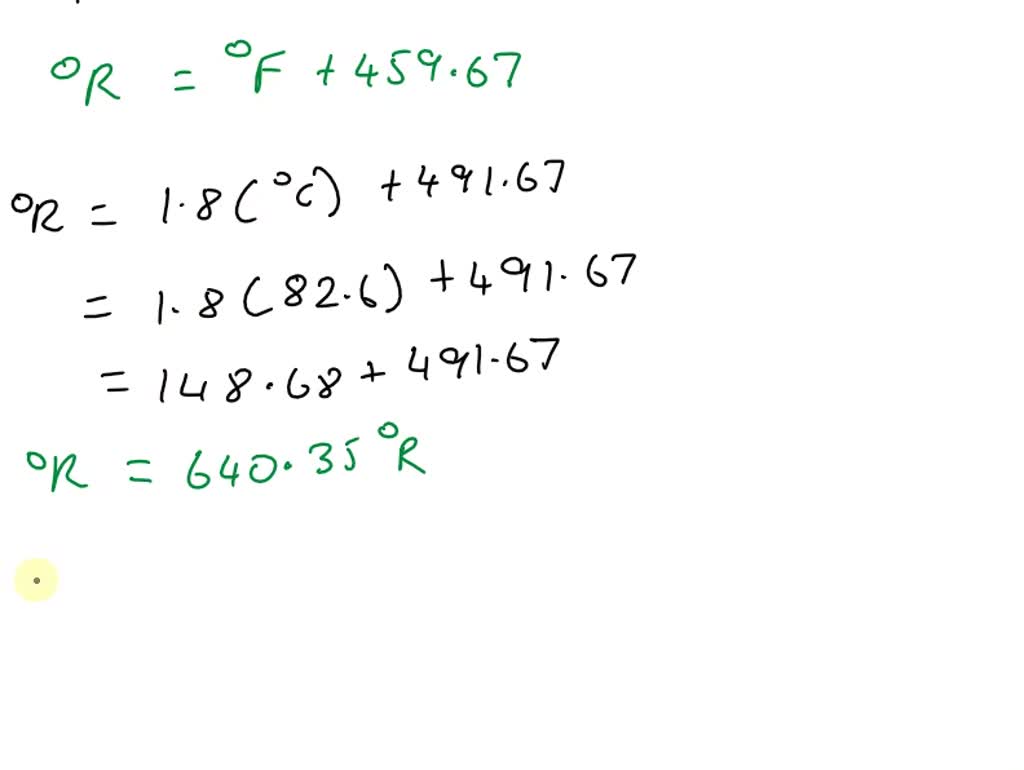
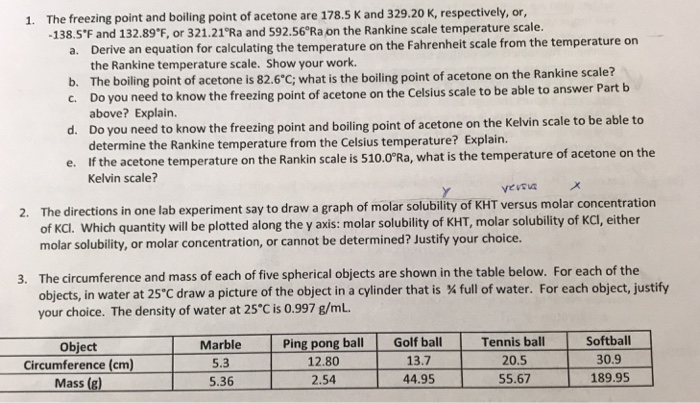
SOLVED: The freezing point and boiling point of acetone are 178.5 K and 329.20 K, respectively, or -138.5°F and 132.89°F, or 321.21°Ra and 592.56°Ra on the Rankine scale temperature scale. Derive an