Study characteristics for CheckMate 227 Part 1A, KEYNOTE-021 Cohort G,... | Download Scientific Diagram

CheckMate 227: 6-year data on the treatment of locally advanced NSCLC with Opdivo and Yervoy - BestPractice Nordic
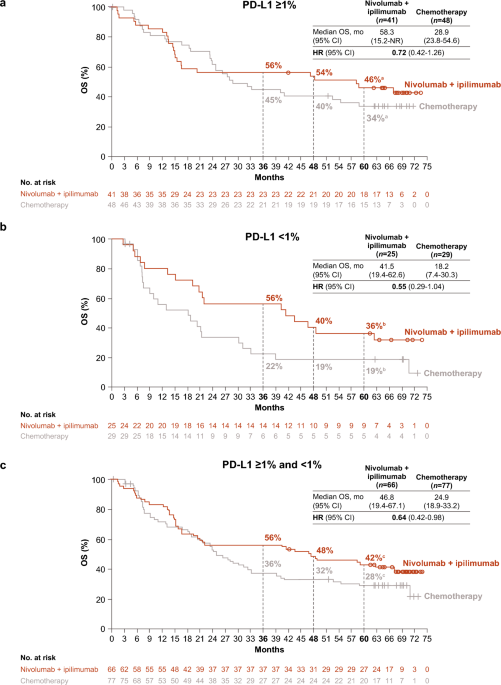
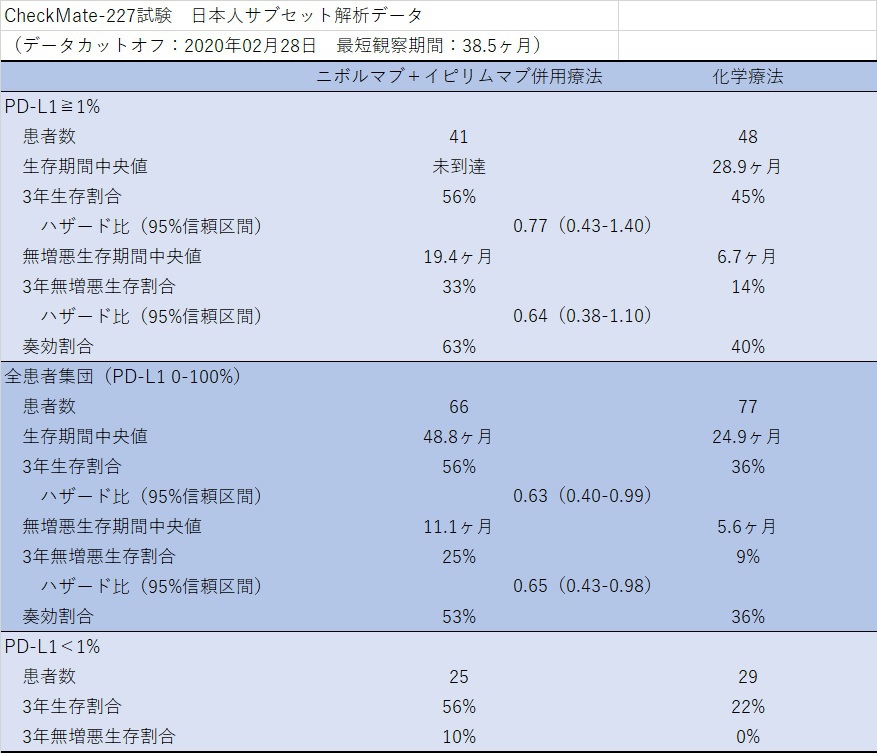
First-line nivolumab plus ipilimumab in metastatic non-small cell lung cancer: 5-year outcomes in Japanese patients from CheckMate 227 Part 1 | International Journal of Clinical Oncology
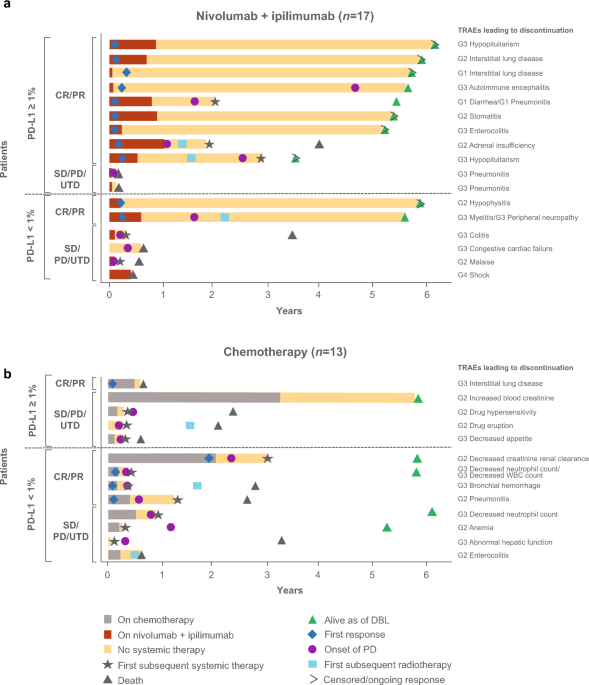
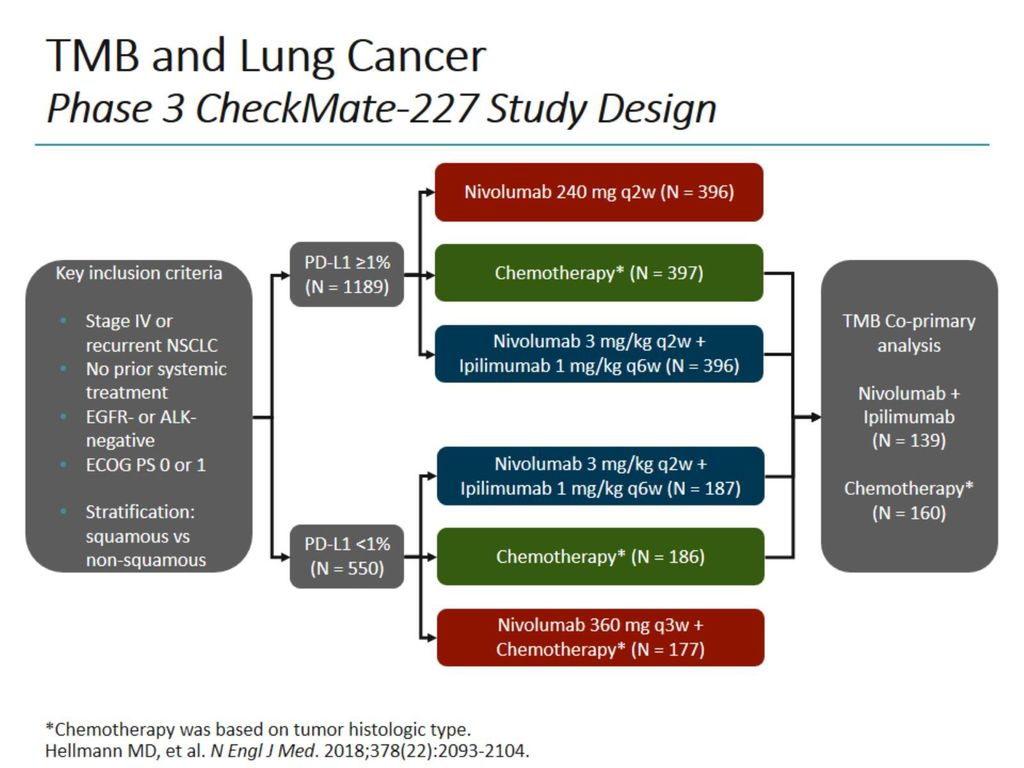
First-line nivolumab plus ipilimumab in metastatic non-small cell lung cancer: 5-year outcomes in Japanese patients from CheckMate 227 Part 1 | International Journal of Clinical Oncology
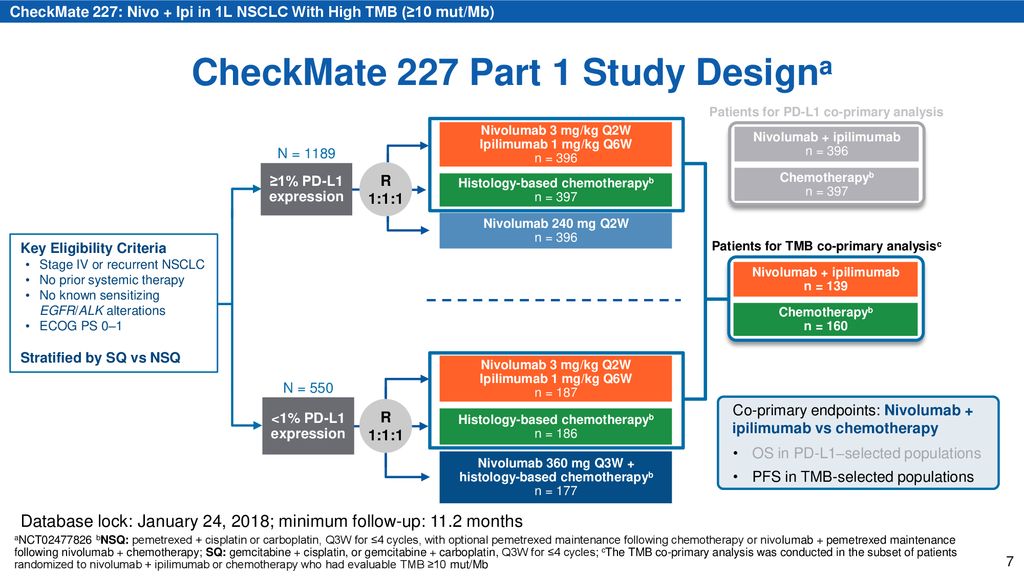
Nivolumab + Ipilimumab vs Platinum-Doublet Chemotherapy as First-line Treatment for Advanced Non-Small Cell Lung Cancer: Initial Results From CheckMate. - ppt download

First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial - ScienceDirect
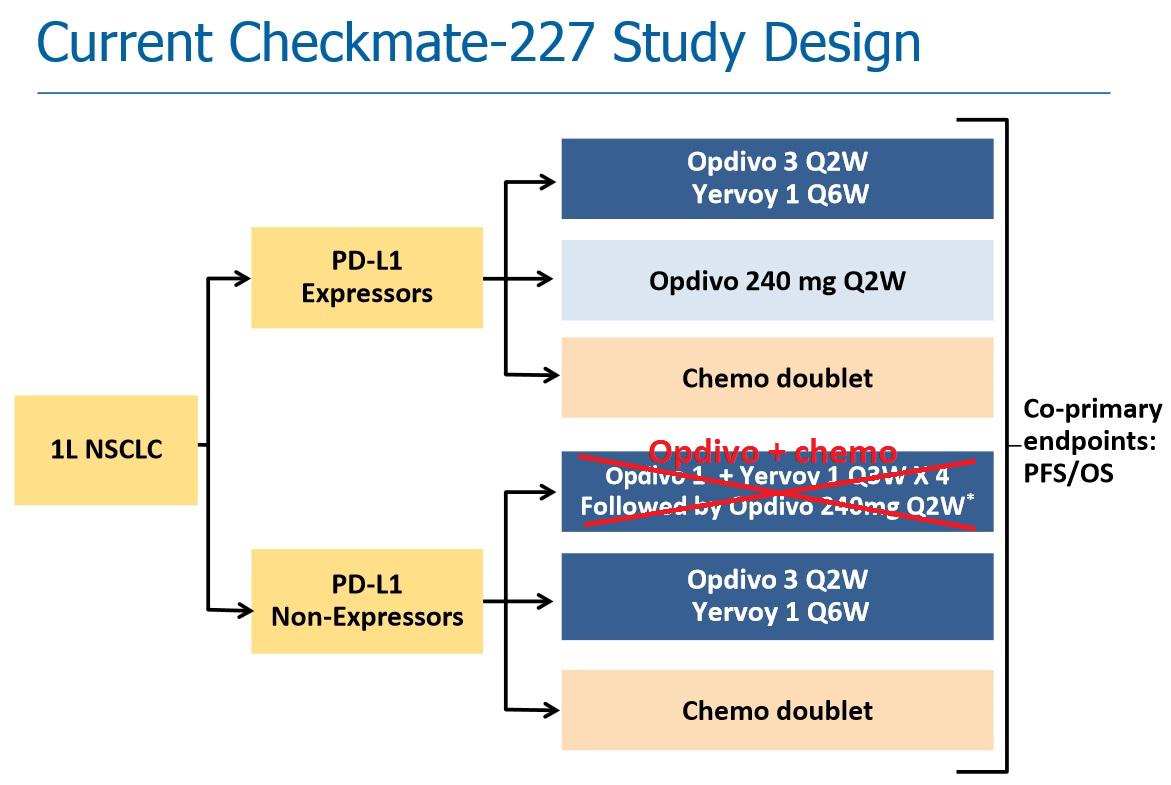
Jacob Plieth on X: "So new $BMY Checkmate 227 design should look sth like this (cf $RHBBY atezeo + chemo #WCLC2015) http://t.co/KKGULGxhEY" / X

Nivolumab + Ipilimumab vs Platinum-Doublet Chemotherapy as First-line Treatment for Advanced Non-Small Cell Lung Cancer: Initial Results From CheckMate. - ppt download

Comparisons between the CheckMate 227 trial and simulation, overall... | Download Scientific Diagram

First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial - ScienceDirect

Nivolumab plus chemotherapy in first-line metastatic non-small-cell lung cancer: results of the phase III CheckMate 227 Part 2 trial - ESMO Open

First-Line Nivolumab Plus Ipilimumab Versus Chemotherapy in Advanced NSCLC With 1% or Greater Tumor PD-L1 Expression: Patient-Reported Outcomes From CheckMate 227 Part 1 - ScienceDirect
![CheckMate 227]nivolumab联合低剂量ipilimumab或nivolumab联合化疗或单纯化疗一线治疗晚期非小细胞肺癌 CheckMate 227]nivolumab联合低剂量ipilimumab或nivolumab联合化疗或单纯化疗一线治疗晚期非小细胞肺癌](https://www.qitaijk.cn/public/uploads/images/20181006/1538805891305306.jpg)